While this is not particularly important for the purification of water this principle is used in the process of freeze drying an important commercial process. Non-electrolytes dont dissociate when they dissolve.

Chapter 13 States Of Matter Ppt Download
Greater than one atmosphere the boiling point of the liquid is greater than its normal boiling point.

. Less than one atmosphere the boiling point of the liquid is lower than its normal boiling point. Where m is the molality of the solution Kb is the molal boiling point elevation constant for the solvent and i is a number related to the number of particles the solute contributes to the solution the vant Hoff i factor. Decreasing the pressure the boiling point decrease.
As the pressure of the gas above the liquid goes down the boiling point temperature also goes down. -The boiling point of a substance increases as the external pressure increases. The boiling point of a liquid increase when.
Water has a boiling point elevation constant of 0512 degrees Cmolal where molality is the concentration unit of moles solute particles per kilogram of solvent. If the boiling point of water is increased when the external pressure is increased then decreasing the external pressure should decrease the boiling point. Because atmospheric pressure can change based on location the boiling point of a liquid changes with the external pressure.
Increasing the pressure the boiling point is increased. An increase in atmospheric pressure raises the boiling point of a liquid by raising the vapor pressure of the water above the liquid. The amount the boiling point is elevated is determined using the equation.
Hence a higher temperature is required to change liquid to gas phase. Effect of Pressure on Waters Boiling Point. A liquid in a high pressure environment boils at a higher temperature.
The pressure is a factor that affects the boiling point of the liquid. At any given time some of the solute molecules will be at the surface thereby reducing the number of solvent molecules chemists say the activity of the solvent decreases relative to the pure solvent at the surface and reducing the rate of evaporation and therefore the vapor pressure all temperatures. The extent of the boiling point elevation can be calculated.
From the p-T graph its evident that as liq-gas boundary bears a positive slope on increasing the pressure the boiling point will increase for water. Effect of PRESSUTE against Boiling point. The higher the air pressure the harder it is for the liquid to evaporate.
So boiling point of liquid rises on increasing pressure. How does air pressure affect boiling point. What effect does the lowering of the external pressure has on the boiling point.
List two application of distillation. Calculating Boiling Point Elevation. Water at high altitudes such as Denver boils at a lower temperature than at sea level.
For the vapor pressure to equal the atmospheric pressure a higher temperature is required and a higher boiling point is observed. Equal to one atmosphere the boiling point of a liquid is called the normal boiling point. The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure.
As this pressure decreases the boiling point of the water also decreases. This increases the amount of thermal energy needed to increase the vapor pressure of the water to match raising the boiling point. -Boiling occurs when the vapor pressure of the liquid is sufficient for bubbles of.
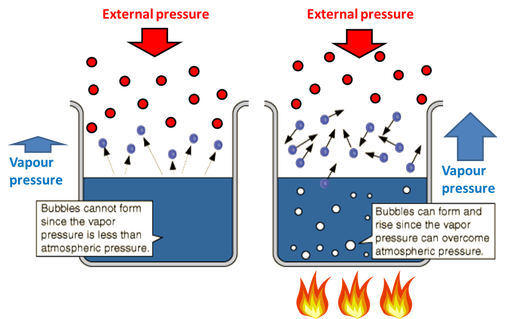
It is directly proportional to the molal concentration of the solution. ΔT b iKbm. -The boiling point is the temperature at which the vapor pressure equals the external pressure.
The normal boiling point is a constant because it is defined relative to the standard atmospheric pressure of 760 mmHg or 1 atm or. State three requirements that must be satisfied before a compound can be steam distilled. As the pressure of the gas above the liquid goes up the boiling point temperature also goes up example.
Apart from simple and fractional distillation list two other distillation techniques available to the organic chemist. When the external pressure is. -The boiling point of a substance increases as the external pressure increases-The boiling point is the temperature at which the vapor pressure equals the external pressure-Boiling occurs when the vapor pressure of the liquid is sufficient for bubbles of vapor to form in the interior of the liquidNotes-A liquid can evaporate at any temperature.
Therefore the boiling point of a solvent or liquid is affected by the atmospheric pressure and boiling point is raised. When placed in a lower pressure environment it boils at a lower temperature. For instance atmospheric pressure up on a high mountain might be somewhere around 08 atmosphere.
The behavior can be explained as I mentioned about gaining sufficient energy to break through the intermolecular bonds. At higher altitudes the atmospheric pressure decreases. The boiling point increases with pressure increase the link below shows a chart of water and the effect of pressure on the boiling point.
Helpful 0 Not Helpful 0. Since the boiling point is defined as the temperature at which the vapor. Therefore if you increase the pressure decreasing the volume then the reaction will progress towards the direction where gas volume DECREASES which is towards liquid phase.
The boiling point of the liquid is lower than the normal boiling point if the external pressure is less than one atmosphere. The formula for boiling point elevation is. As the pressure applied to the liquid surface is increased the energy needed for the liquid molecules to expand to gas phase also increases.
The boiling point of a solution then will be greater than the boiling point of the pure solvent because the solution which has a lower vapor pressure will need to be heated to a higher temperature in order for the vapor pressure to become equal to. Overall the entire apparatus increases the vapor pressure on the gas gas pressure on the external pressure which increases the boiling point.
Unit 3 Mixtures And Pures Substances Colligative Properties San Francisco De Paula Science Department

Boiling Point External Pressure Liquid And Gases Chapter No 4 Chemistry Part 1 Youtube

What Is The Relationship Between Boiling Point And Vapour Pressure Quora
0 Comments